
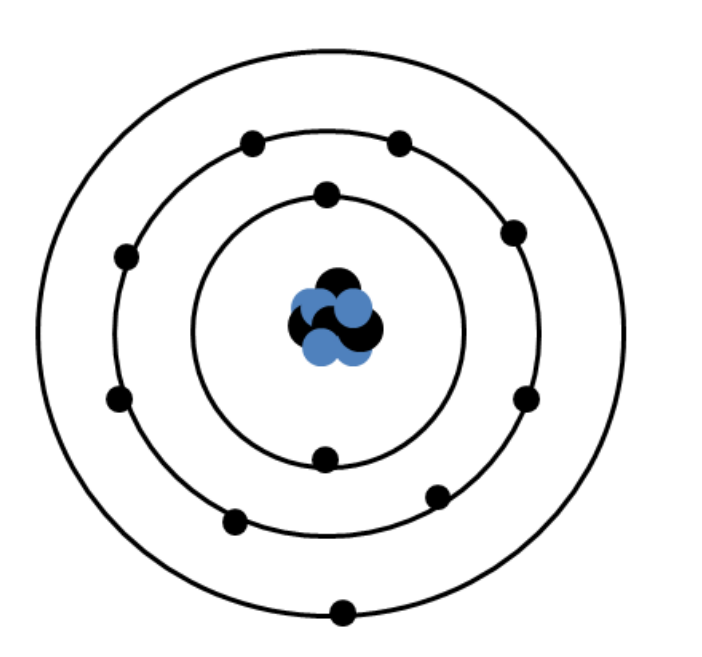
Atoms can achieve this more stable state by having a valence level which contains as many electrons as it can hold. From this perspective, bonds between atoms form so that the bonded atoms are in a lower energy state compared to when they were by themselves. Octet of Valence ElectronsĪtoms gain, lose, or share electrons in their valence level in order to achieve greater stability, or a lower energy state. In the case of gold, there is only one valence electron in its valence level. Chemical reactivity of all of the different elements in the periodic table depends on the number of electrons in that last, outermost level, called the valence level or valence shell. The outermost principal energy level is of great interest in chemistry because the electrons it holds are the furthest away from the nucleus, and therefore are the ones most loosely held by its attractive force the larger the distance between two charged objects, the smaller the force they exert on each other. The second principal energy level can have 8, the third can have 18, and so on, until all 79 electrons have been distributed. The first principal energy level, which is the one closest to the nucleus, can hold a maximum of two electrons. Notice that the outermost level has only one electron.Īs an example, a neutral atom of gold (Au) contains 79 protons in its nucleus and 79 electrons. The number of electrons in each level is listed on the upper right corner of the figure. Notice that the first energy level (closest to the nucleus) can have only two electrons, while more electrons can ‘fit’ within a given level further out. Principal energy levels of gold (Au): The figure shows the organization of the electrons around the nucleus of a gold (Au) atom.

We say the electrons ‘reside’ in different principal energy levels, and these levels exist at different radii from the nucleus and have rules regarding how many electrons they can accommodate. Careful investigations have shown that not all electrons within an atom have the same average position or energy.

The electrostatic attraction between them keeps electrons ‘bound’ to the nucleus so they stay within a certain distance of it. Principal Energy LevelsĪn atom consists of a positively charged nucleus and negatively charged electrons. These Lewis symbols and Lewis structures help visualize the valence electrons of atoms and molecules, whether they exist as lone pairs or within bonds. Lewis structures (also known as Lewis dot structures or electron dot structures) are diagrams that represent the valence electrons of atoms within a molecule. Lewis symbols (also known as Lewis dot diagrams or electron dot diagrams) are diagrams that represent the valence electrons of an atom.

Electrons exist outside of an atom ‘s nucleus and are found in principal energy levels that contain only up to a specific number of electrons.


 0 kommentar(er)
0 kommentar(er)
